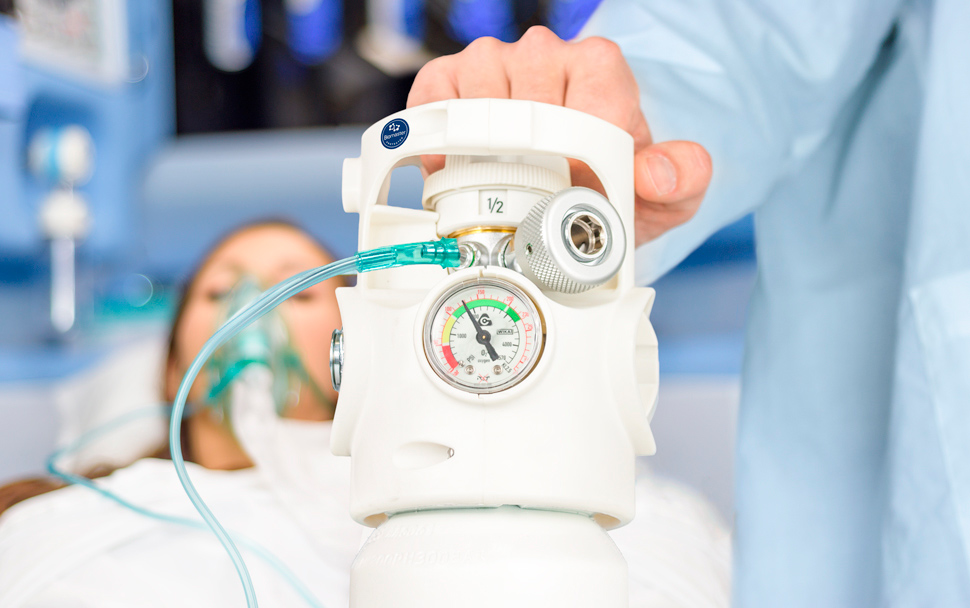
Thanks to the Cavagna Group’s Viproxy Biomaster antibacterial technology, it is now possible to protect handles on medical gas cylinder from the risks of bacterial surface contamination.
– Ponte San Marco (Brescia – Italy), November 9th, 2015 – Did you know that medical gas cylinders are particularly exposed to the risk of bacterial contamination, especially oxygen tanks? Indeed, they are among the most touched, handled and transported of all medical instruments. In particular, the handle is exposed to the hazards of bacterial cross-contamination. Most of the time, cleaning and disinfecting is simply not enough. There are many dangerous situations that can occur in health care facilities and during the performance of paramedical services, be it in hospital wards, emergency rooms, hospices, pharmacies or cylinder handling. Yet at the same time, even the patient’s own home presents numerous occasions for bacterial contamination to build up, such as contact with nurses providing home care to the chronically ill and the elderly, family members who personally tend to their loved ones, assistive personnel providing services at home, or people who are merely paying a visit to a sick person.
For all these reasons, the handle on the Cavagna Group’s Viproxy valve has adopted the innovative protection that only Biomaster antibacterial technology can provide; yet another first for the Cavagna Group, which has reaffirmed its leading role as a manufacturer of technological solutions for the regulation and control of gas worldwide. The application of Biomaster protection technology to the Viproxy handle is the result of a partnership between the Cavagna Group and Biomaster ADD UK, a British company that specializes in incorporating silver ions into all kinds of materials and products in order to make them antibacterial. Biomaster ADD has pioneered the development of permanent antibacterial protection around the world, and it is a recognized leader in antimicrobial additives for polymers and coatings.
By incorporating this special silver ion technology into its polymers, the Viproxy Biomaster inhibits the growth of harmful bacteria and makes the plastic parts of its handle completely safe to contact. Whether in health care facilities or at the patient’s home, it provides permanent, around-the-clock antibacterial protection for the lifetime of the product, no matter how the cylinder is handled.
For thousands of years, silver has been known and appreciated for its natural antimicrobial properties; indeed, it has been used and developed to prevent the growth of bacteria, while presenting no toxic effects on humans.
Biomaster provides rigorously tested protection: in fact, silver and nanoparticles are successfully used as antimicrobial protection in a wide range of industrial applications.
Biomaster silver ion technology is even effective against several species of antibiotic-resistant bacteria, such as Staphylococcus aureus (MRSA) and Vancomycin-resistant Enterococcus (VRE), as well as E.coli and Legionella. Biomaster antimicrobial protection acts by binding itself to the cell wall and disrupting DNA replication in the bacteria; yet while it is merciless to bacteria, it remains harmless to human beings. Biomaster protection complies with the latest ISO standards and is frequently subject to strict quality control. In addition, this antimicrobial system has been approved by the FDA and the EPA. Biomaster protection is incredibly durable, long lasting and highly active for the entire lifetime of the product.
Biomaster Protection acts in the following places:
- on the cell surface – The silver ions bind themselves to the cell surface of the bacteria, destroying the cell wall and preventing its regeneration;
- in the enzymes – The silver ions are attracted to the bacteria’s enzyme cells, where they inhibit the energy produced by the bacterium itself.
- in the DNA chain – The silver ions disrupt the DNA of the bacterium’s cell; in this way they prevent DNA replication and the creation of new cells.
The Cavagna Group’s Viproxy incorporates a shut-off valve and pressure regulator into a single block.
About the Cavagna Group
The Cavagna Group has been supplying industries and consumers worldwide since 1949, and it is today’s leading manufacturer of equipment and fittings for compressed gases, gas storage and gas control. The Cavagna Group offers the most complete and reliable line of products in the sector, as well as the most dynamic services.
Both the experience and reliability of the Group’s product range have led to long-lasting partnerships with virtually all major oil/gas companies, in addition to producers of compressed gas containers and gas appliance OEMs. The Cavagna Group employs approximately 1,000 people worldwide. The Group is made up of nine vertically integrated production companies in Italy and nine other companies spread out across five continents. With a distribution network consisting of an additional fifteen fully-owned distribution companies, the Cavagna Group now sells its products in more than 135 countries worldwide. For more info, please visit: www.cavagnagroup.com
AT A GLANCE
Each year there are over 3 million cases of hospital-acquired infections in Europe.
Research conducted by the Stockholm-based European Centre for Disease Prevention and Control (ECDC) found that on any given day, one in 18 patients acquires an infection in a health care facility, amounting to about 3.2 million cases a year.
Infections represent an increasing threat to health care environments, especially those stemming from antibiotic-resistant bacteria such as MRSA. However, the majority of these infections could be avoided, if only hospitals would implement some additional measures of prevention and control. For this reason, the European Centre for Prevention and Disease Control has urged hospital administrations to strengthen the fight against bacterial contamination.
The above-mentioned survey involved 1,000 health care facilities in 30 European countries, and the number of hospital-acquired infections was estimated to be 15 thousand. The main causes of the infections were found to be Klebsiella pneumoniae, a gram-negative bacterium that causes pneumonia, and fecal bacteria, such as Escherichia coli (or E. coli). Another bacterium found in the survey was Staphylococcus aureus, better known as MRSA because of its acquired resistance to the antibiotic Methicillin.
It is believed that MRSA is the cause of infection for about 53 million people worldwide every year. According to a research made by Royal College of Physicians every case of MRSA costs the National Health Service an extra £9,000. It is estimated that the total cost to the NHS has exceeded £1billion.
Hospital-acquired infections such as MRSA are the most common complications during hospital stays, as well as one of the most serious problems for patient safety.









